Bernards' Injection Molding Tips, Techniques and Technology for Shop Floor Technicians and Supervisors | home
SHOP FLOOR TIPS | MORE TIPS | MORE TIPS STILL | SAFETY | TROUBLESHOOTING | RHEOLOGY | DE-COUPLED MOLDING | MOLD FLOW ANALYSIS | MOLD CHANGE FORM | SHOP FLOOR RULES | WATT-WATTCHER | MORE PATENTS | QUESTIONS/COMMENTS
RHEOLOGY
UNDER CONSTRUCTION
RHEOLOGY OF PLASTICS

RHEOLOGY IS JUST A FANCY WORD THAT MEANS "THE WAY PLASTICS FLOW" HOWEVER, BEFORE I CAN EXPLAIN THE CONCEPTS, YOU WILL NEED TO LEARN ABOUT THE BASIC BUILDING BLOCKS OF THE UNIVERSE!

ATOMS
Any substance that cannot be decomposed into simpler substances by ordinary chemical processes is a chemical element. Ninety-two elements occur in nature either chemically free, such as oxygen, or, as in the case of water, in combination with other elements. Twenty additional elements have been produced artificially.
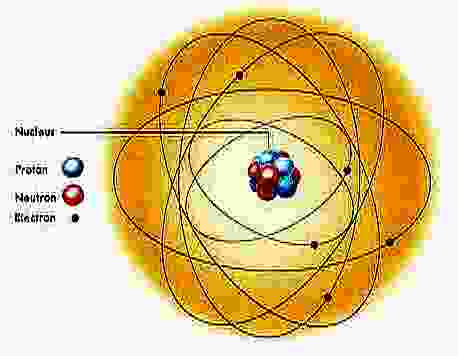
Atoms form the building blocks of the simplest substances, the chemical elements. Familiar elements include hydrogen, oxygen, carbon, and lead. Each element consists of one basic kind of atom. Atoms vary greatly in weight, but they are all about the same size. For example, an atom of plutonium, the heaviest element found in nature, weighs more than 200 times as much as an atom of hydrogen, the lightest known element. However, the diameter of a plutonium atom is only about 3 times that of a hydrogen atom.
If this hydrogen atom were drawn to scale, you would need a computer
screen a mile wide to display the electron's orbit!

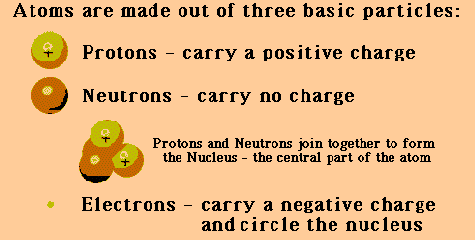
Tiny as atoms are, they consist of even more minute particles. The three basic types are protons, neutrons, and electrons. Each atom has a definite number of these subatomic particles. The protons and neutrons are crowded into the nucleus, an exceedingly tiny region at the center of the atom. If a hydrogen atom were about 4 miles (6.4 kilometers) in diameter, its nucleus would be no bigger than a tennis ball. The rest of an atom outside the nucleus is mostly empty space. The electrons whirl through this space, completing billions of trips around the nucleus each millionth of a second. The fantastic speed of the electrons makes atoms behave as if they were solid, much as the fast-moving blades of a fan prevent a pencil from being pushed through them.

Atoms are often compared to the solar system, with the nucleus corresponding to the sun and the electrons corresponding to the planets that orbit the sun. This comparison is not completely accurate, however. Unlike the planets, the electrons do not follow regular, orderly paths. In addition, the protons and neutrons constantly move about at random inside the nucleus.
MOLECULES
The smallest units into which a compound can be divided without changing its physical and chemical properties are molecules. They are formed from combinations of atoms, the basic building blocks of all matter Because molecules consist of various combinations of the more than 100 kinds of known atoms, they exist in a very large number of structural forms. Molecules can form rings or chains. They can join in very long repeating structures known as polymers. In certain conditions, they can form a very precise crystal lattice. In general, the structural design of a molecule will favor the most energetically stable combination of bonds among the atoms.
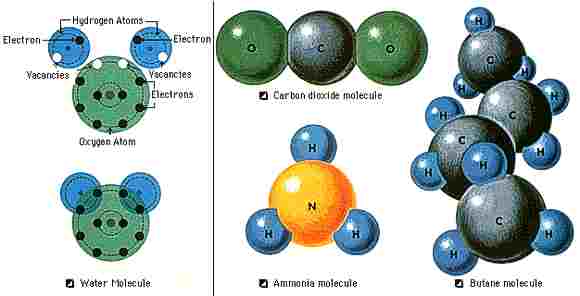
A molecule is not static but dynamic and constantly in motion. Its atoms rotate and vibrate around one another. The positions of the atoms, their relative motions, and their bond strengths can be changed by heat, magnetic fields, and other forces. Whether a substance will be solid, liquid, or gas under ordinary conditions depends upon the nature of the chemical bonds and the relative freedom of movement of the atoms within the molecules.
Atoms link together in molecules through strong attractive forces called bonds. The shape of a molecule depends upon two factors: (1) The atoms tend to take up positions relative to one another such that the bonds formed are the strongest of all the bonds that this particular group of atoms could form. (2) Atoms that are not bonded to each other tend to move far apart.
When atoms combine to form a molecule they either share or exchange electrons. True molecular compounds are formed when two or more atoms share electrons, resulting in what is called a covalent bond. These bonds are very strong and require a good deal of energy to break. Compounds formed with covalent bonds do not conduct electricity, and they are referred to as nonpolar because they are not very soluble in water.
Polar molecules, on the other hand, are readily soluble in water. They are formed when atoms exchange an electron to form a bond called an ionic bond. These bonds are strong because the atoms have become more stable as a result of either losing or gaining an electron (called ionization). Molecules formed from ionic bonds are not true molecules because the electrons are not shared but exchanged.
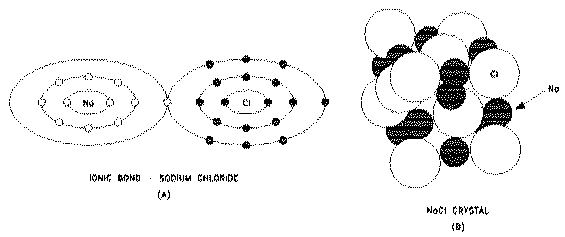
Compounds formed by ionic bonding are made up of a random mixture of ions--atoms or groups of atoms that carry an electric charge as a result of having lost or gained electrons. They retain a charge because they are not bonded to the particular atom with which they exchanged electrons. An example is sodium chloride (NaCl), or common table salt. Sodium gives up an electron and becomes positively charged (Na+) when heated in the presence of chlorine. This electron is acquired by chlorine, leaving it with a net negative charge (Cl-). The resultant water-soluble NaCl is a collection of free ions that are capable of conducting an electrical current.
Molecules are made up of atoms held together in certain arrangements. Scientists use chemical formulas to show the composition of molecules. For example, a water molecule consists of two hydrogen atoms and one oxygen atom, and it has the formula H2O. A molecule's size and shape depends on the size and number of its atoms. A molecule that consists of two atoms, such as nitric oxide (NO), is called a diatomic molecule. A molecule made up of three atoms, such as water, is called a triatomic molecule. A large molecule, such as DNA, can contain millions of atoms.
AND FINALLY
POLYMERS!
Plastics are polymers. What is a polymer? The simplest definition of a polymer is something made of many units. Think of a polymer as a chain. Each link of the chain is the "mer" or basic unit that is made of carbon, hydrogen, oxygen, and/or silicon. To make the chain, many links or "mers" are hooked or polymerized together. Natural polymers include such things as tar and shellac, tortoise shell and horns, as well as tree saps that produce amber and latex. Natural polymers began to be chemically modified during the 1800s to produce many materials. The most famous of these were vulcanized rubber, gun cotton, and celluloid. The first synthetic polymer produced was Bakelite in 1909.
Many common classes of polymers are composed of hydrocarbons. These polymers are specifically made of small units bonded into long chains. Carbon makes up the backbone of the molecule and hydrogen atoms are bonded along the backbone. Below is a diagram of polyethylene, the simplest polymer structure.
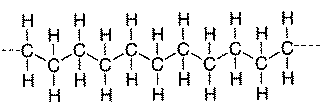
There are polymers that contain only carbon and hydrogen (for example, polypropylene, polybutylene, polystyrene, and polymethylpentene). Even though the basic makeup of many polymers is carbon and hydrogen, other elements can also be involved. Oxygen, chlorine, fluorine, nitrogen, silicon, hosphorous, and sulfur are other elements that are found in the molecular makeup of polymers. Polyvinyl chloride PVC) contains chlorine. Nylon contains nitrogen and oxygen. Teflon contains fluorine. Polyesters and polycarbonates contain oxygen. Vulcanized rubber and thiokol contain sulfur. There are also some polymers that, instead of having carbon backbones, have silicon or phosphorous backbones. These are considered inorganic polymers. One of the most famous silicon-based polymers is Silly Putty.
Polymerization and Molecular Structure
The initial compound that is used to form polymers is the "mer" or monomer. Monomers are chemically joined together in one of two ways: addition polymerization or condensation polymerization.
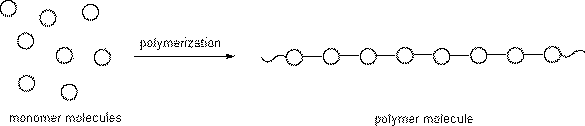
Addition polymerization is comprised of three basic steps: initiation, propagation, and termination. For example, during the initiation phase of the polymerization of polyethylene, the double bonds in the ethylene "mers" break and begin to bond together. A catalyst or promoter may be necessary to begin or speed up the reaction. The second phase, propagation, involves the continued addition of monomers together into chains.
The final step is termination. During termination all monomers may be used, causing the reaction to cease. A polymerization reaction can cease by quenching the reaction. Similar to quenching someone’s thirst, water can be used to quickly cool a reaction. Polymers formed by addition polymerization include acrylic, polyethylene, and polystyrene, to name a few.
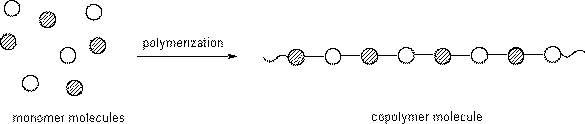
Copolymers are made when more than one type of monomer is used for the reaction.
Very simply, addition polymerization describes the process of "mers" joining by each one adding on to the end of the last "mer." A simple visual of the process is paper clips joined together to form a long chain. Polymers formed by addition polymerization are often thermoplastic in nature. Thermoplastics are like hot melt glue sticks that can be heated and made soft and then become hard when cooled. Thermoplastic polymers are easily processed and reprocessed or recycled. The majority of polymers used today are thermoplastics.
The other group of polymers are formed by condensation polymerization. During the chemical reaction of condensation polymerization, a small molecule is eliminated as the monomers join together. Common polymers in this group include nylons, some polyesters, urea formaldehyde, and urethanes. These polymers can be thermoplastic in nature or thermosetting. Once a thermoset polymer is formed, it cannot be melted and reformed. All plastics flow at some time during their processing and are solid in the finished state, but once a thermoset is processed, it is dramatically different and cannot be reformed.
The means of polymerization will affect the heat reaction of the formed polymer; likewise, the arrangement of the "mers" within the molecule will affect the physical characteristics of the formed polymer. "Mers" joined together in long chains have a linear configuration very similar to a paper clip chain, even though in actuality tetrahedral bonds give the molecule a zigzag arrangement. During polymerization, if the "mers" not only form straight chains but also form long side chains off the main backbone, the resulting configuration is described as branched, like a tree branch or grape stem. A third configuration is achieved by the long chains being chemically linked together. An example would be natural rubber (isoprene) being reacted with sulfur. The sulfur bonds the chains to form a giant meshwork molecular structure that is known as vulcanized rubber. This is a cross-linked configuration.
Polyethylene has the simplest "mer" structure. Even though the backbone of other polymers will be similarly formed by a broken bond between two carbons, the remaining carbons in the "mer" will form a functional group whose orientation about the backbone will affect the physical nature of the resulting polymer. For example, propylene is the "mer" that will form polypropylene:
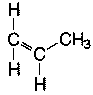
Polymerization will be initiated by the double bond breaking and the "-mers" joining together. Therefore, the methyl group on the propylene "-mer" has the potential to be located at various points along the backbone. If the methyl group (CH3) is oriented repeatedly on one side of the chain on alternating carbons, it is called isotactic.
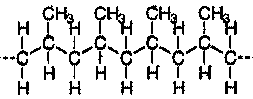
Ninety to nine-five percent of all polypropylene polymers have this configuration.
THE RHEOLOGY OF POLYMERS
NON-NEWTONIAN FLOW
Rheology is the study of the flow and deformation of matter from solids to gases. It describes the relationship between force, deformation and timeand the term comes from the Greek "rheos" that means to flow. The consistency of different products describes fluid rheology. Fluid rheology is studied by viscosity and elasticity. Viscosity is the resistance to flow and elasticity is usually stickiness.Common to liquids, solids and substances in between the former two is that if a stress is applied to them, they will strain. Stress may be visualized by placing a small amount of fluid between two parallel plates. When one plate slides over the other, forces act on the fluid dependent upon the rate of the plate movement. This causes a shear stress on the liquid.
Are you totally confused? Let me first explain some terms. Viscosity is the resistance to flow. For example, think about motor oil. A low viscosity of, say, 10 weight, is fairly thin and flows relatively easily. On the other hand, a high viscosity of 30 weight is rather thick and resists flow. Elasticity is the property whereby a solid material changes its' shape under opposing forces and tends to recover its' shape when the force is removed. A good example of elasticity is found in a rubber band. Stretch it and it changes its' shape. Let go and it recovers. All liquids that have a simple molecular formula and low molecular weight, such as water, etc., are said to exhibit non-Newtonian flow. What this means is that as the shear rate (speed of flow) increases, the viscosity (resistance to flow) does not change. Ten weight oil is always ten weight oil (at a given temperature) no matter how fast it is being pumped thru your engine. Water always has the same "thickness". Now let me give you an example of non-Newtonian flow at its' extreme: Silly Putty. Hold a ball of Silly Putty in your hand and slowly squeeze it. You are actually applying a shear force. Does it resist that force? No, not much. It acts like it has a relatively low resistance to flow, or in other words a low viscosity, and it oozes between your fingers. Now take that ball of Silly Putty, place it on a table and hit it with a hammer. What happened? It shattered! Silly Putty is non-Newtonian. Its' viscosity increases with increases in shear rates. When the shear is low, as when you squeezed it, its' viscosity is low and it does not resist flow. However, when you struck it with a hammer you applied a high shear----you tried to make it flow quickly and it resisted. In fact, it resisted so much that it acted like a solid and it shattered.
Plastics are considered to exhibit non_Newtonian flow. However, they flow opposite of the silly Putty. As shear rates increase, plastics resist flow less! To put it in more understandable terms, as injection speed increases the melt viscosity decreases and the plastic flows easier. Now why would this be? As you recall from the section above, polymers are long chains of molecules. When they are at rest or moving slowly they look something like this:
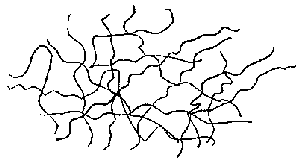
Each of those squiggly lines represent a polymer molecule. As you can see, there is no alignment and they are mixed together haphazardly. However, something unusual begins to happen as you shoot them into the mold. The faster you move them, or in other words, the faster you inject them the more they begin to orient themselves in a more or less straight line. The figure below depicts this:
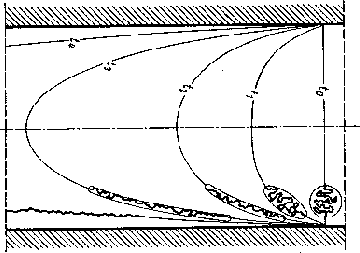
As the molecules align themselves in a straighter line, the resistance to flow decreases. The faster you inject the material, the more aligned they become and it becomes easier and easier to push them into the mold. This is one reason that higher injection speeds are preferable to slower speeds.
Plastics also share another property that non-Newtonian materials do not. Plastics are compressible. The two figures below depict the non-compressibility of water and the compressibility of polymers.
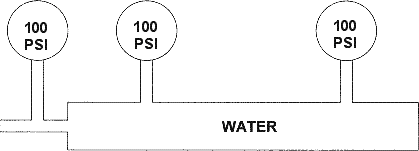
NOTE THAT THE PRESSURE IS EQUAL AT ALL POINTS
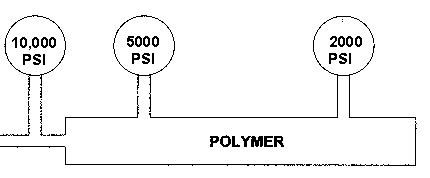
NOTE THE SIGNIFICANT PRESSURE DROPS
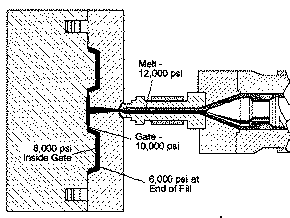
TYPICAL PRESSURE DROPS IN A MOLD
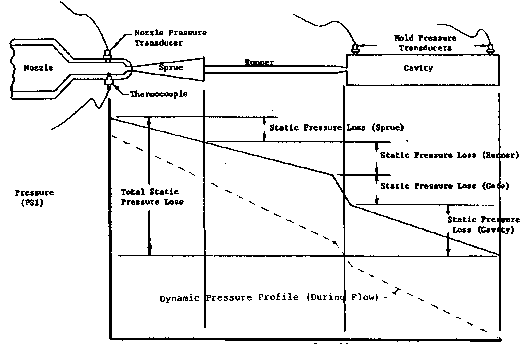
GRAPHIC REPRESENTATION OF PRESSURE DROPS

UNDER CONSTRUCTION